XPS MultiQuant |
Application Examples |
The soda-lime glass samples were etched with hydrochloric acid and heat treated 550 K in air. Then samples were silylated was with hexamethyl-disiloxane vapour in a sealed glass ampoule.
The acid treatment performed under moderate conditions leads to the complete depletion of Na, K, Ca and a partial depletion of Al to a depth far beyond the detection limit of XPS. The heat treatment did not stimulate reappearance of K and Ca in the surface layers to levels above the detection limit.
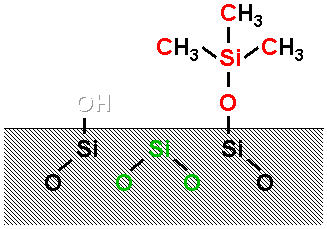
Two types of carbon atoms were found: the hydrocarbon type from the methyl groups and a H-C-O type contamination. Two types of oxygen and silicon could be distinguished. The smallest peaks can be identified as those originating from the supposed (CH3)3-Si-O type surface species, while the larger ones originate from the oxygen and silicon atoms of the glass surface network.

| Peak | Binding energy (eV) |
Composition (atomic %) |
Origin |
|---|---|---|---|
| C1s | 286.1 | 2.4 | contamination |
| 284.1 | 11.5 ( 3 ) | -CH3 | |
| O1s | 533.2 | 46.7 ( 2 ) | Si-O-Si, Si-OH |
| 531.5 | 3.6 ( 1 ) | (CH3)3Si-O- | |
| Si2p | 103.8 | 27.2 ( 1 ) | Si-O-Si, Si-OH |
| 102.2 | 3.9 ( 1 ) | (CH3)3Si- |
As it can be seen, the atomic % values for the (CH3)3SiO-group show an excellent consistency with the expected 3:1:1 ratio. The binding energy values are in good accordance with the literature. The ratio of the corresponding bulk Si and O atoms is also close to 2, which means that OH groups can hardly be found on the silylated surface.
I. Bertóti, M. Mohai, M. Révész, G. Alexander:
Surface Composition of Glasses: Modifications Induced by Chemical and Heat Treatments.
Vacuum 37(1-2), 129 (1987).